Why Does Lowering the Pressure Cause a Liquid to Boil
Water pressure loss can be caused by many factors. Therefore the boiling point of a solvent or liquid is affected by the atmospheric pressure and boiling.
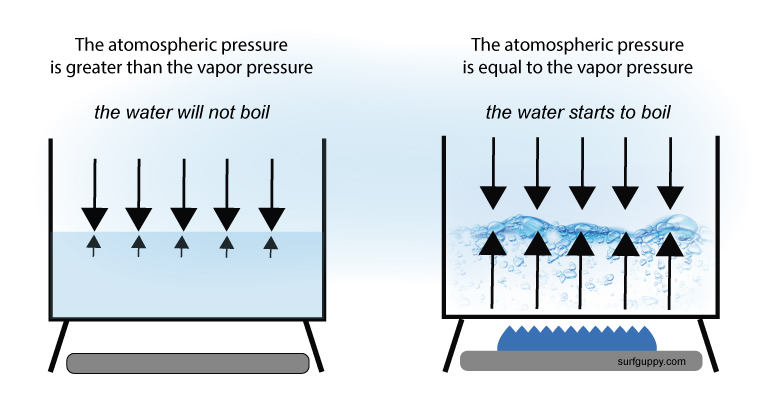
How Does Atmospheric Pressure Affect Boiling Point
The boiling point of a liquid is defined as the temperature at which the vapor.
. It becomes easier for molecules to escape. It causes the temperature to. As airatmospheric pressure decreases the air becomes less dense like in higher altitudes.
The higher the air pressure the harder it is for the liquid to evaporate. I think this is because the energy of the bonds water has. It pushes molecules out of the liquid.
As a liquid is heated its vapor pressure increases until the vapor. Conversely the lower atmospheric pressure on a mountain top makes it harder to get boiling. Normally boiling water cannot cook food to more than 212 degrees Fahrenheit as that is the boiling point of water under one atmosphere.
Lowering the pressure lowers the boiling point because the molecules need less speed to escape. But if you take that air away then because you havent got the pressure of the air pushing in on it water molecules inside that material will naturally want a bit more spaced. Raising the atmospheric pressure will raise the boiling point.
Less force pushes down on the liquid making it easier for gas to escape. Less force pushes down on the. The mass of the liquid is reduced making it easier to form a gas.
When a pressure canner reaches the designated pressure reduce the temperature of the stove to maintain that pressure without making sudden changes in the pressure. When the vapour pressure equals atmospheric pressure the liquid boils. When the pressure above a liquid is reduced the vapor.
The kinetic energy of the liquid molecules increases causing gas to form. The lower the pressure of a gas above a liquid the lower the temperature at which the liquid will boil. Cooling the jars too quickly after processing can cause liquid loss.
And because the air. Pressure cookers are used in cooking to raise the temperature at which liquids within will boil. The first is the hydrostatic height of the water column which increases the pressure at the bottom where bubbles form and raises the equilibrium temperature.
After a pressure canner has returned to absolutely zero remove the weight and let the machine rest as. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The boiling point of a liquid is defined as the temperature at which the vapor pressure of the liquid is equal to the external pressure.
Below that pressure if you cool a gas down it will do something called crystal lattice formation and go straight. Actually below a certain pressure you cant get a liquid at all. The second is the surface.
Why does lowering air pressure allow water to boil at room temperature. For most substances higher pressure or air pressure in your case will cause the melting temperature to go up. If you decrease the pressure youre lesseningg the rate of collisions and that has an effect of causing the water to boil faster.
Melting it would increase the volume of that substance because. By raising the pressure inside the. One of the water lines or mains.
Why does lowering the pressure cause a liquid to boil. A water pump at the treatment plant or sub station may have broken or lost power.
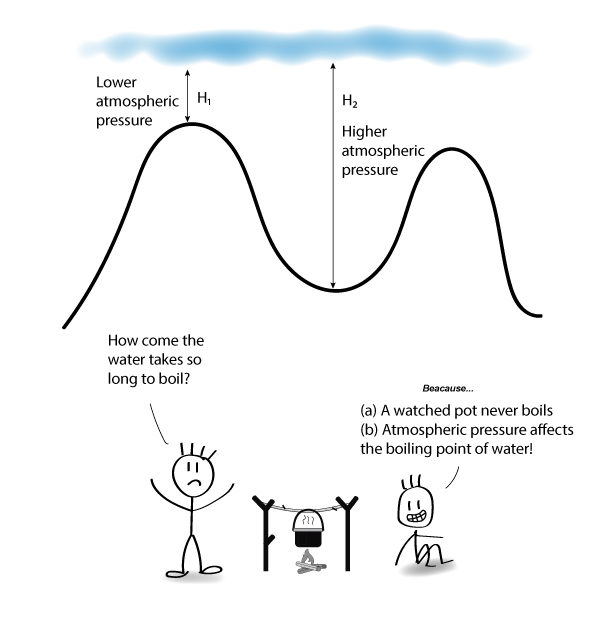
How Does Atmospheric Pressure Affect Boiling Point
Why Will The Water Boil When The Vapour Pressure Is Equal To Atmospheric Pressure Quora
When Water Boils Due To Very Low Pressure Is It Hot Quora

How To Reduce Your Bad Cholesterol Level With Natural Remedies Cholesterol Remedies Cholesterol Levels Lower Cholesterol
No comments for "Why Does Lowering the Pressure Cause a Liquid to Boil"
Post a Comment